
Although little known outside the nuclear industry, dysprosium titanate–an inorganic ceramic compound—is ideal for use in control rods in nuclear reactors and as a host material for nuclear waste. Among its desirable properties, the element dysprosium is a great thermal neutron absorber, and dysprosium titanate has low thermal expansion, large heat capacity, a high melting point, suppression of outgassing, low swelling under irradiation, and chemical compatibility with cladding materials.
Under extreme irradiation conditions with massive, highly charged, high-energy atoms called swift heavy ions, nuclear materials often undergo changes at the atomic level, which affect many of their physical properties.
This is why NE’s Pietro F. Pasqua Fellow and Associate Professor Maik Lang and his research colleagues set out to understand the radiation response of two dysprosium titanate oxides (Dy2TiO5 and Dy2Ti2O7) across different length scales—from the specific atomic arrangements to the average crystal structure. Their findings were recently published in npj Materials Degradation.
In ordered materials, the atomic arrangement of crystalline solids—where atoms, ions, and molecules are arranged in a predictable, specific pattern—repeats itself at distances that are many atoms away and form extensive, “long-range” order. However, in amorphous, non-crystalline solids, atoms have a more random arrangement and their positions become uncorrelated after only a short distance.
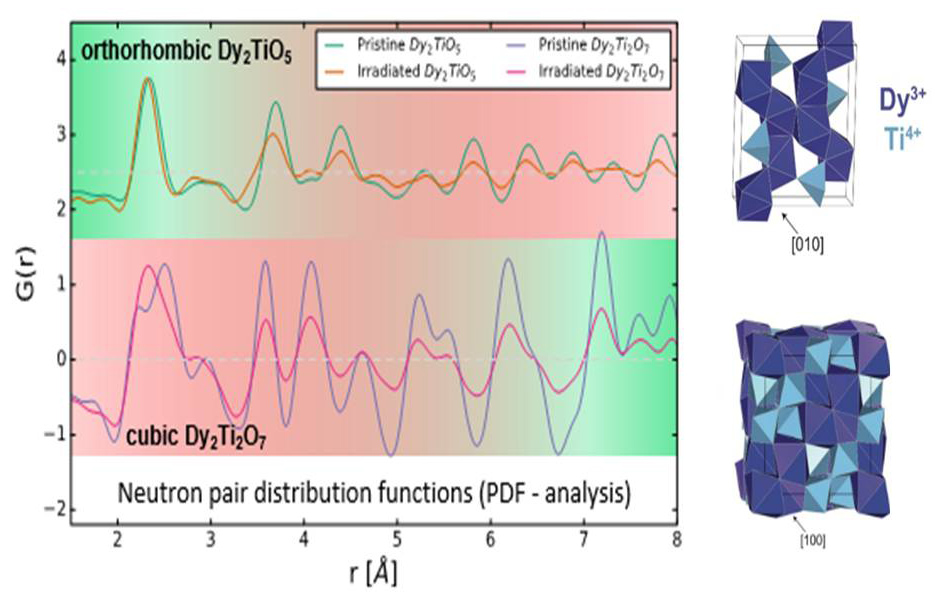
Neutron pair distribution function analysis shows evolution of radiation resistance (green equals more resistant) across length scales; the two titanate oxides have opposed behavior, and while Dy2Ti2O7 is more resistant to amorphization (bottom, large r-values) Dy2TiO5 retains largely its local atomic bonding environment (top, small r-values).
Crystalline solids scatter X-rays in specific directions that form a distinct pattern, called a diffraction pattern, that can be analyzed to describe their structure. Amorphous solids, which lack long-range structure, only diffusely scatter X-rays and form indistinct diffraction patterns that cannot be easily analyzed. At distances spanning only a few atoms, however, the atoms are still bonded to each other in a specific, ordered manner that can be investigated using techniques other than standard X-ray diffraction pattern analysis.
To understand long-range amorphization caused by ion irradiation, and the atomic-scale order in the irradiated ceramics, the research team used neutron total scattering experiments at the Spallation Neutron Source at Oak Ridge National Laboratory with pair distribution function analysis. This technique is similar to X-ray diffraction, but allows the user to better investigate the local atomic bonding environment.
The team discovered that the dysprosium titanate which is more readily amorphized (Dy2TiO5) retains its local atomic bonding environment while the material that better retains crystallinity (Dy2Ti2O7) undergoes significant changes at the atomic scale that involves the movement of the atoms into a different bonding arrangement.
This study demonstrates that a comprehensive assessment of radiation behavior should be performed at all length scales to capture the structural complexity and heterogeneity present in many irradiated nuclear materials, suggesting that the definition of radiation resistance depends on the length scale of interest.
In this specific case, Dy2Ti2O7 would be the material of choice to retain crystallinity to much higher ion fluences, while Dy2TiO5 is superior in maintaining a more stable, homogenous atomic-bonding environment with less atomic rearrangements through the entire amorphization process.